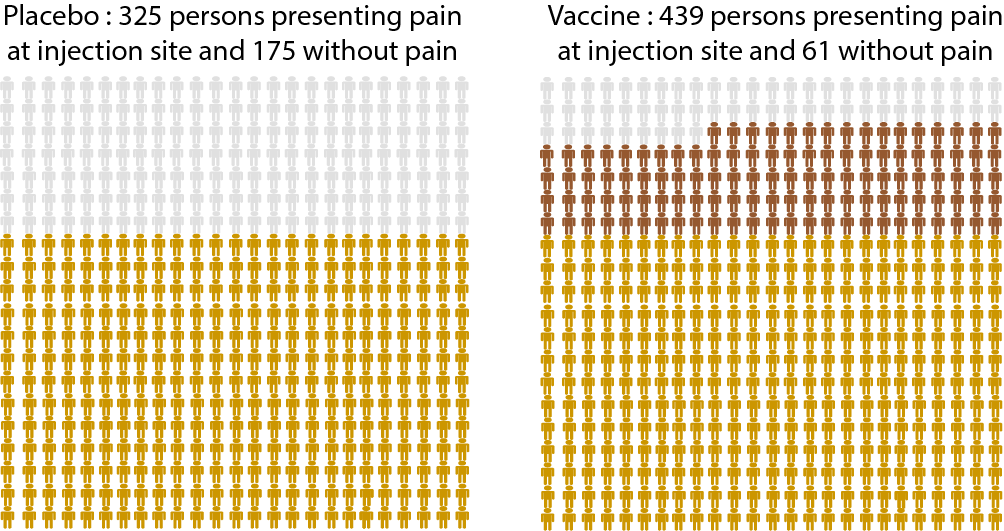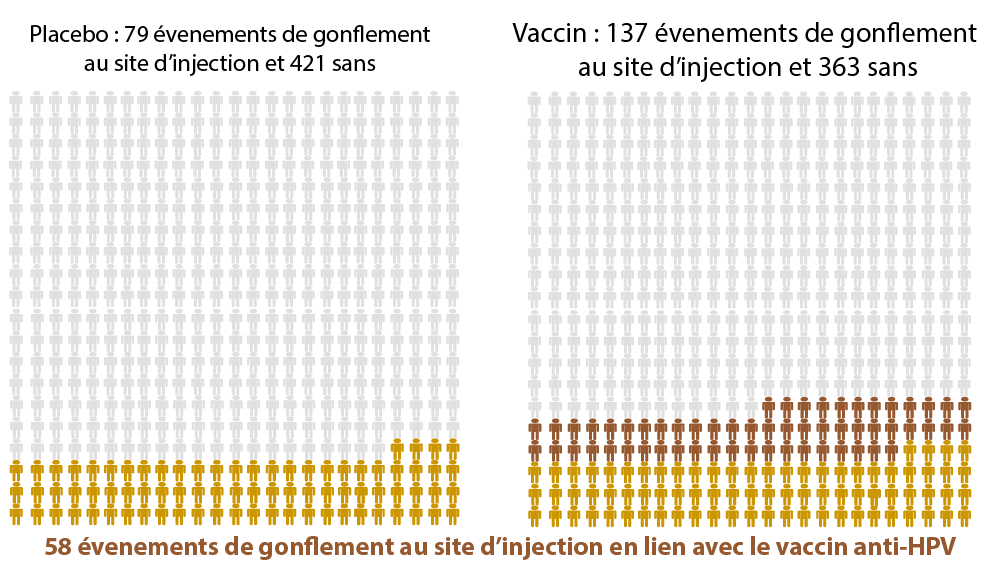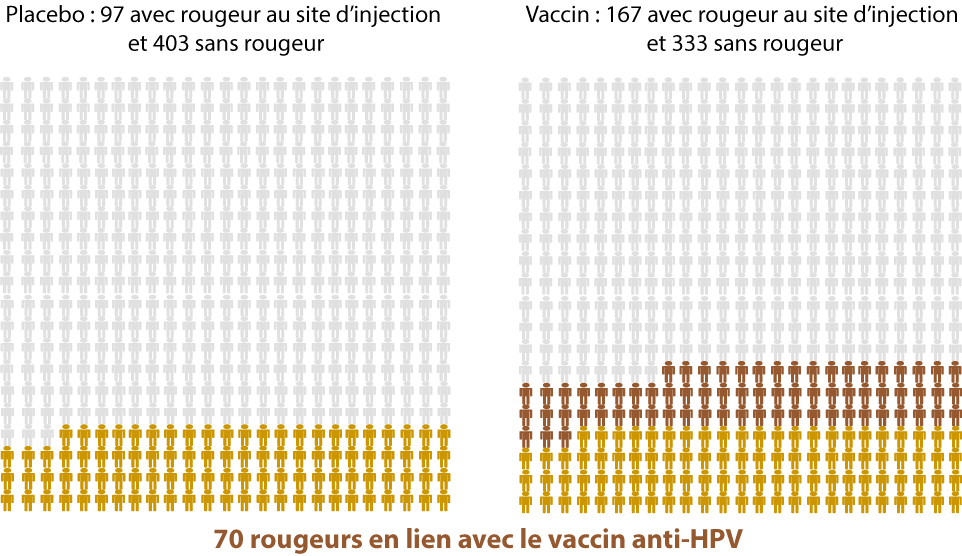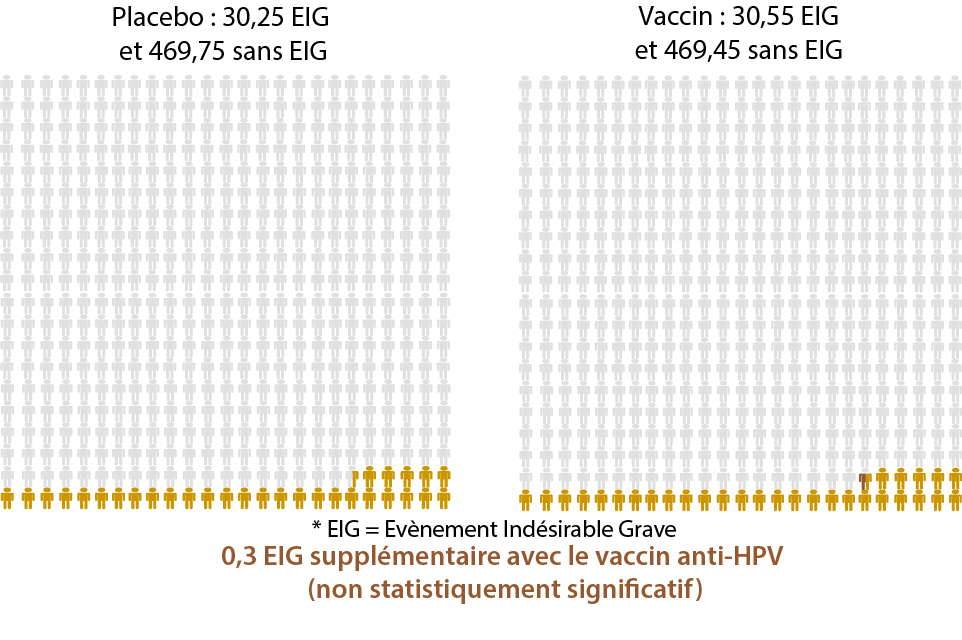Nonvalidated english version
The human Papillomavirus (HPV), what is it ?
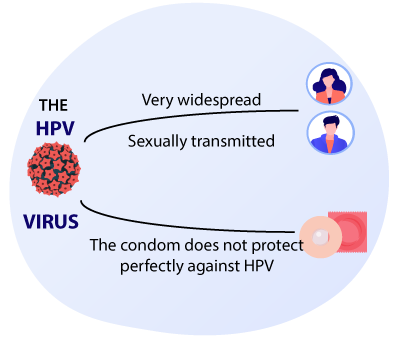
The Human PapillomaViruses (HPV) are a very common group of viruses. It is a sexually transmitted infection for which wearing a condom only partially protects. Both men and women can be infected with HPV.
Key data regarding HPV infections
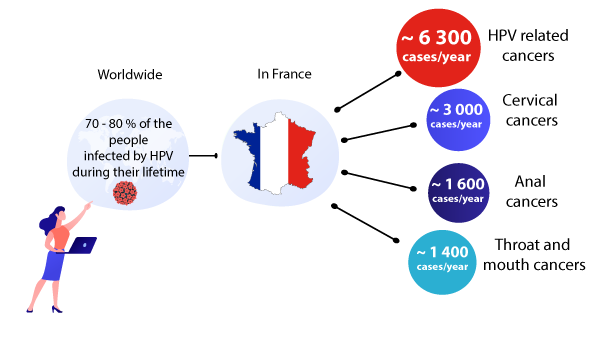
It is estimated that 70% to 80% of sexually active people will be infected with HPV at least once in their life. According to the National Cancer Institute, more than 6,300 cancers per year were HPV-related in 2015.
The most common HPV-related cancer is cervical cancer. In France, there are nearly 3,000 new cases of cervical cancer per year and 1,100 deaths per year are due to this cancer. Another common HPV-related cancer is anal cancer (about 1600 cases each year) (1).
HPV related diseases
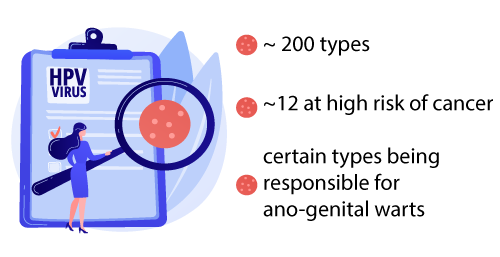
There are about 200 different types of this virus. Most of the time, the human body spontaneously eliminates the virus, but in nearly 10% of cases, the infection persists and can lead several years later to precancerous lesions which can then progress to cancer.
A dozen types are “high risk” oncogenic viruses, that is to say viruses that can be responsible for cancer.
Other strains can give benign genital warts. Most of the time, the human body spontaneously eliminates the virus, but in nearly 10% of cases, the infection persists and can lead several years later to precancerous lesions which can then progress to cancer (1).
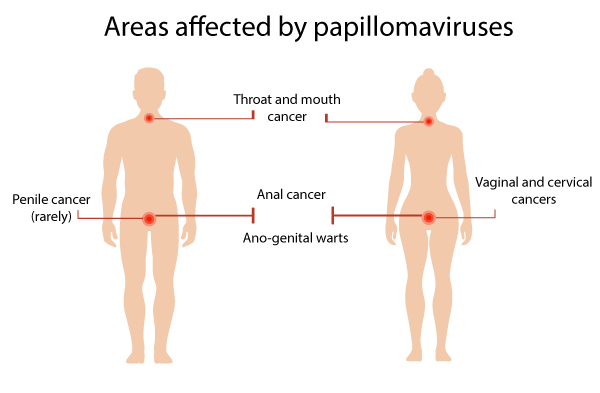
What are the areas that could be affected by the HPV virus?
There are several types of HPV-related cancers:
- Cervical cancer
- vulvar cancer
- anal cancer
- mouth and throat cancer
- penile cancer (more rarely)(2)
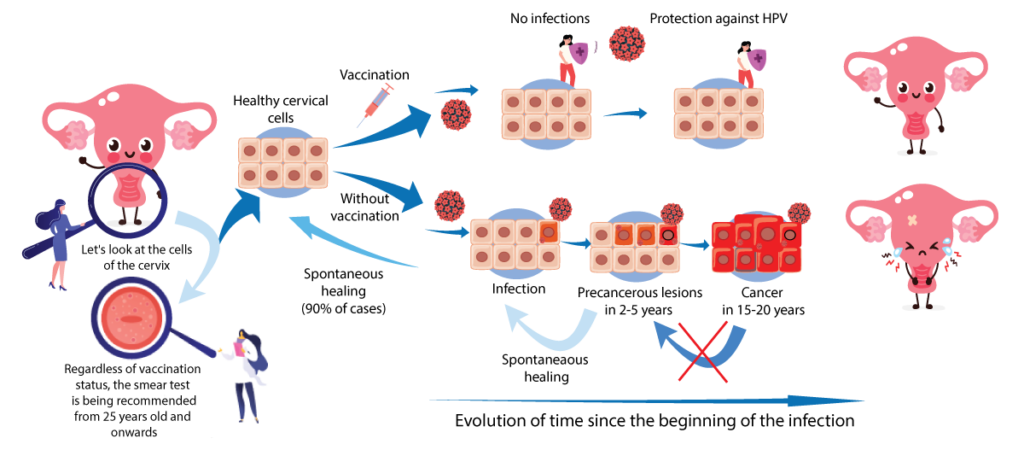
Let's see the example of the cervical cancer
Vaccination and HPV infections
-
Vaccination against human papillomaviruses prevents infections by the HPV strains concerned by the vaccine. The vaccine is most effective when given before exposure to the virus.
-
90% of cases of HPV-induced cancers are due to the types targeted by the nine-valent vaccine (HPV 6, 11, 16, 18, 31, 33, 45, 52, 58) (3).
-
The vaccine called GARDASIL® nonavalent has been recommended since 2018 following the quadrivalent GARDASIL® (6, 11, 16,18) recommended since 2007.
-
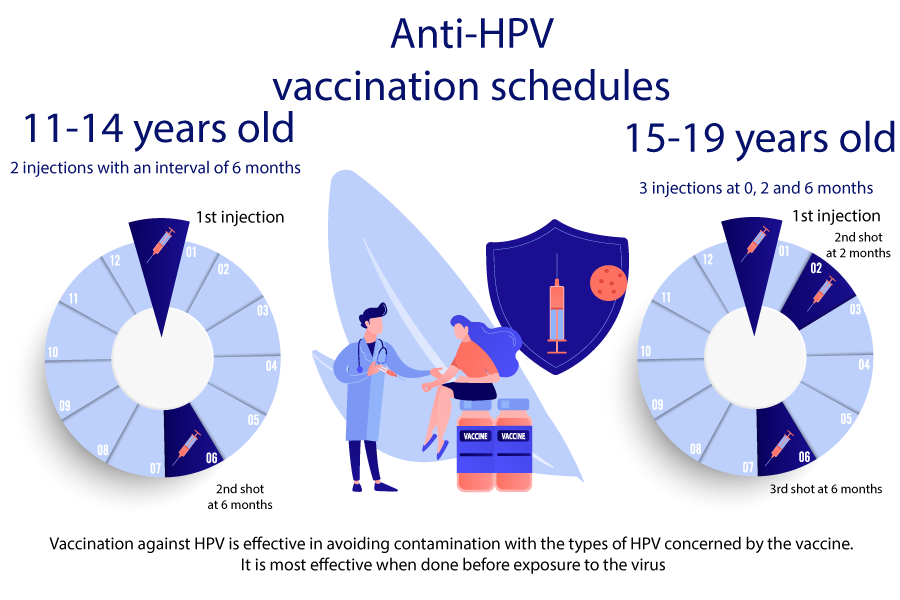
In France, the vaccine is intended for all young girls and all young boys from 11 to 14 years old with possible catch-up up to 19 years old.
-
Two injections are to be performed between 11 and 14 years (at 0 then 6 months later).
-
From the age of 15, 3 injections will be required (at 0, 2 and 6 months).
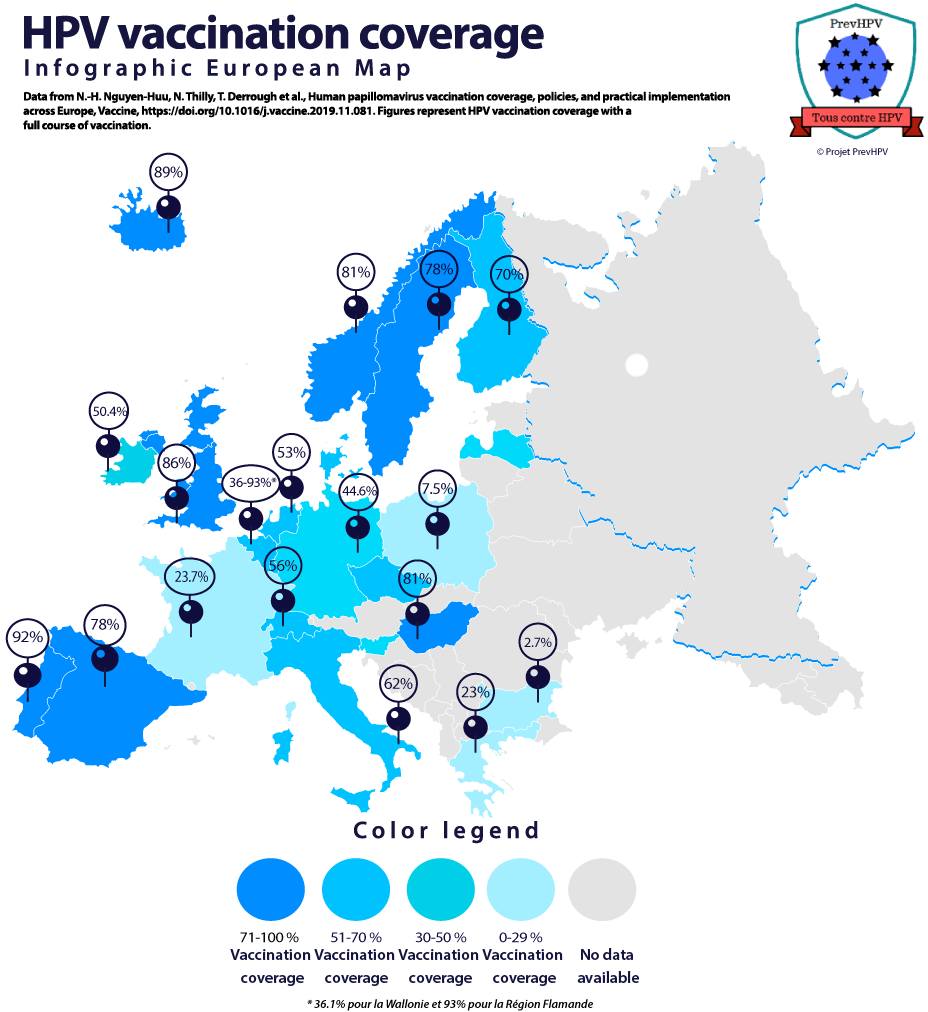
Vaccine coverage in Europe (4,17)
Vaccine effectiveness
Impact of vaccination on precancerous lesions of the cervix:
It takes several decades to see the effectiveness of the vaccine on the appearance of cancer, however studies have already shown the effectiveness of the vaccine on precancerous lesions of the CIN2+ type (5)

8.5 lesions avoided thanks to vaccination
Protection against cervical cancer
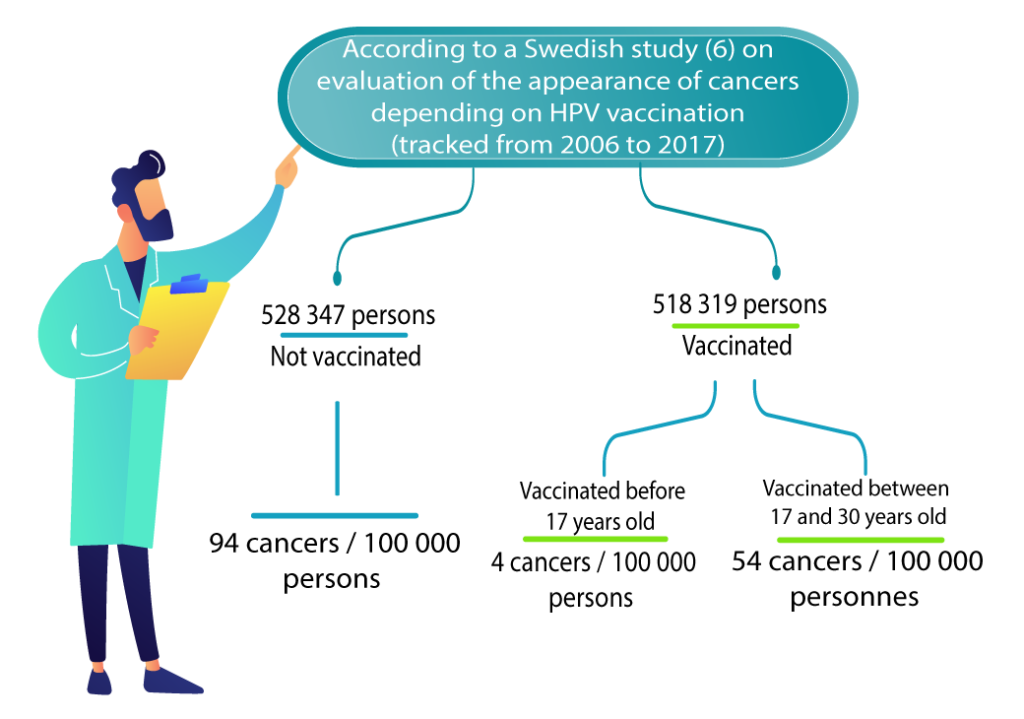
A Swedish study followed 1,672,983 women aged 10 to 30 from 2006 to 2017. This study showed a decrease in the number of new cases of cervical cancer among vaccinated women. This follow-up is still short for the appearance of cancer but it is the first study showing a signal of vaccine efficacy on cervical cancer (6) . Another Danish cohort study shows similar figures, with a decrease in cumulative incidence from 11.3/100,000 in the unvaccinated to 6.7/100,000 in the vaccinated (vaccinated mainly between 20 and 30 years old) (19) .
Similar results are also found in an English cohort study published in November 2021. It confirms a reduction in the incidence of cervical cancer, in particular if vaccination coverage is high and if women are offered the vaccine at a younger age (20).
Protection against other HPV related cancers
Data are beginning to be published regarding the decrease in HPV-induced cancers in patients vaccinated against HPV.
The Finnish cancer registry showed protection on all HPV-induced cancers (including vulvar cancer and throat and mouth cancer). There was only 7 years of follow-up so far. It is still too early to have reliable figures, but these data are encouraging (7)
Protection against cervical warts

Anogenital warts (or condyloma) are very common in women as in men (between 94,920 and 117,888 new cases per year in France). They are highly contagious and mainly affect young adults. They are systematically induced by HPV. Types 6 and 11 are found in 90% of condyloma cases. In general, the lesions themselves are not painful, although some patients may experience itching, burning or irritation. Nevertheless, these unsightly lesions recur and their management is particularly long and painful (8).
After 5 to 8 years of vaccination, in real life, there was a decrease in the diagnosis of anogenital warts of 67% for women aged 15 to 19 and 48% for men. In countries with more than 50% vaccination coverage, these figures increase to 88% for women and 86% for men (9).
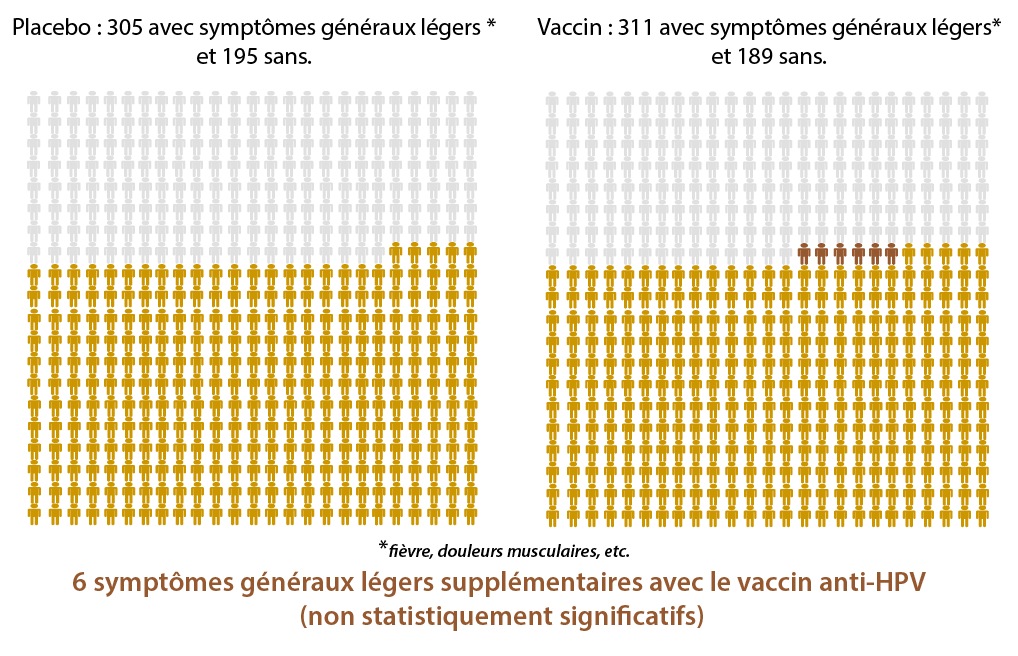
Side effects of the HPV vaccination(5)
Statistically significant differences between a placebo and the HPV vaccine
(= these differences seem to be directly related to the HPV vaccine)
Out of 500 people…
Non-statistically significant differences between a placebo and the HPV vaccine
(= these differences do not seem to be directly related to the HPV vaccine)
Autoimmune diseases
Guillain-Barré Syndrome (GBS) is a rare condition in which the patient’s immune system attacks peripheral nerves. Most people with Guillain Barré syndrome make a full recovery, even in the most severe cases. (10)
Regarding the link between vaccination and GBS, the data are discordant.
A French pharmacoepidemiology study found an increased risk of GBS of the order of 1 to 2 additional cases for 100,000 young girls vaccinated. (11) The link between GBS and HPV vaccine has not been found in several other studies (12,13,18).
The Global Advisory Committee on Vaccine Safety (GACVS) rated the vaccine as « extremely safe » in its July 2017 report. It does not identify GBS as an adverse reaction to the vaccine. (15)
Allergies graves
Risk of anaphylaxis (severe allergy): approximately 1.7 cases per million doses (14)
Think about what might help you make your decision for HPV vaccination. How important is it that you:
Avez-vous apprécié ce contenu ?
[Total: 0 Average: 0]
Fact sheet
The human Papillomavirus (HPV), what is it?
Key data
Vaccine coverage
Areas affected by HPV